Educational
Dec 23, 2025
SBIR vs DARPA for Medical & Biotech Companies

Justin Zloty
Medical and biotechnology companies exploring non-dilutive federal funding often hear the same advice: “Apply for SBIR.” While the Small Business Innovation Research Program (SBIR) is an important entry point into government funding, it is not always the best, or most strategic, option for companies developing breakthrough medical technologies.
As a physician who works directly in government contracting, I regularly advise clients on whether SBIR, DARPA, or another Department of Defense pathway best aligns with their technology, timeline, and long-term commercialization goals. The answer depends less on scientific merit and more on how your innovation fits into federal acquisition strategy.
This article explains the practical differences between SBIR and DARPA funding for medical and biotech firms, with a focus on how to choose the right path, and how to avoid costly misalignment.
Understanding the Strategic Purpose of SBIR
The SBIR program was created to stimulate innovation among small businesses while meeting federal research and development needs. Agencies such as National Institutes of Health, Department of Defense, and others allocate a portion of their R&D budgets to SBIR awards.
In practice, SBIR functions as a structured, phased research pipeline. Phase I evaluates feasibility, Phase II funds further development, and Phase III encourages commercialization using non-SBIR funds. For early-stage medical companies, particularly those emerging from academic research, this structure provides predictability and relatively low compliance friction.
However, SBIR is fundamentally conservative. Review panels tend to favor clearly scoped projects with well-defined technical risk and incremental innovation. Technologies that lack an obvious regulatory or commercial endpoint may struggle, even if they are scientifically novel.
How DARPA Approaches Medical Innovation Differently
DARPA operates on an entirely different philosophy. Rather than funding research for its own sake, DARPA funds capability creation. Its medical and biomedical programs exist to solve operational problems faced by the military, often under extreme conditions involving trauma, neurological injury, infectious disease, or human performance limits.
Unlike SBIR, DARPA does not use a phased small-business framework. Instead, it issues Broad Agency Announcements (BAAs) that solicit solutions to open-ended challenges. Funding decisions are made by program managers with deep technical expertise and broad discretion.
This structure allows DARPA to fund medical concepts that would be considered too risky, too interdisciplinary, or too unconventional for traditional biomedical funding agencies. It also means that DARPA expects proposers to understand defense relevance, acquisition mechanics, and execution risk at a much higher level.
Structural Comparison: SBIR vs DARPA
The following table highlights the most important structural differences medical and biotech firms should consider.
Dimension | SBIR | DARPA |
|---|---|---|
Primary purpose | Early-stage innovation | Strategic capability creation |
Risk tolerance | Moderate | Extremely high |
Funding structure | Phase I/II/III | Program-based milestones |
Typical award size | $150K - $1.5M | $5M–$50M+ |
Proposal format | Standardized | Custom, BAA-driven |
Military relevance | Optional (agency-dependent) | Mandatory |
Commercialization focus | Explicit | Implied but expected |
Commercialization focus | Low to moderate | High |
For companies developing incremental medical devices, diagnostics, or software platforms, SBIR often represents the fastest and least risky funding option. For companies pursuing transformational therapies, advanced neurotechnology, or platform technologies with defense relevance, DARPA may be the only agency willing to fund the work at scale.
Which Path Is Better for Your Medical Company?
The decision between SBIR and DARPA should be driven by technology maturity, ambition, and operational relevance, not by familiarity or convenience.
SBIR is generally better suited for companies that are pre-revenue, closely aligned with academic research, and seeking validation before engaging private investors. It works particularly well for therapeutics and diagnostics that follow established regulatory pathways and address well-characterized clinical problems.
DARPA is better suited for companies whose technology challenges existing paradigms, integrates multiple scientific disciplines, or addresses medical problems in extreme or non-traditional environments. Examples include neural interfaces, regenerative medicine for trauma, battlefield diagnostics, and AI-driven medical decision systems.
Critically, DARPA funding often accelerates downstream opportunities, including follow-on DoD contracts, Other Transaction Authority (OTA) agreements, and private sector partnerships. However, this upside comes with significantly higher expectations and scrutiny.
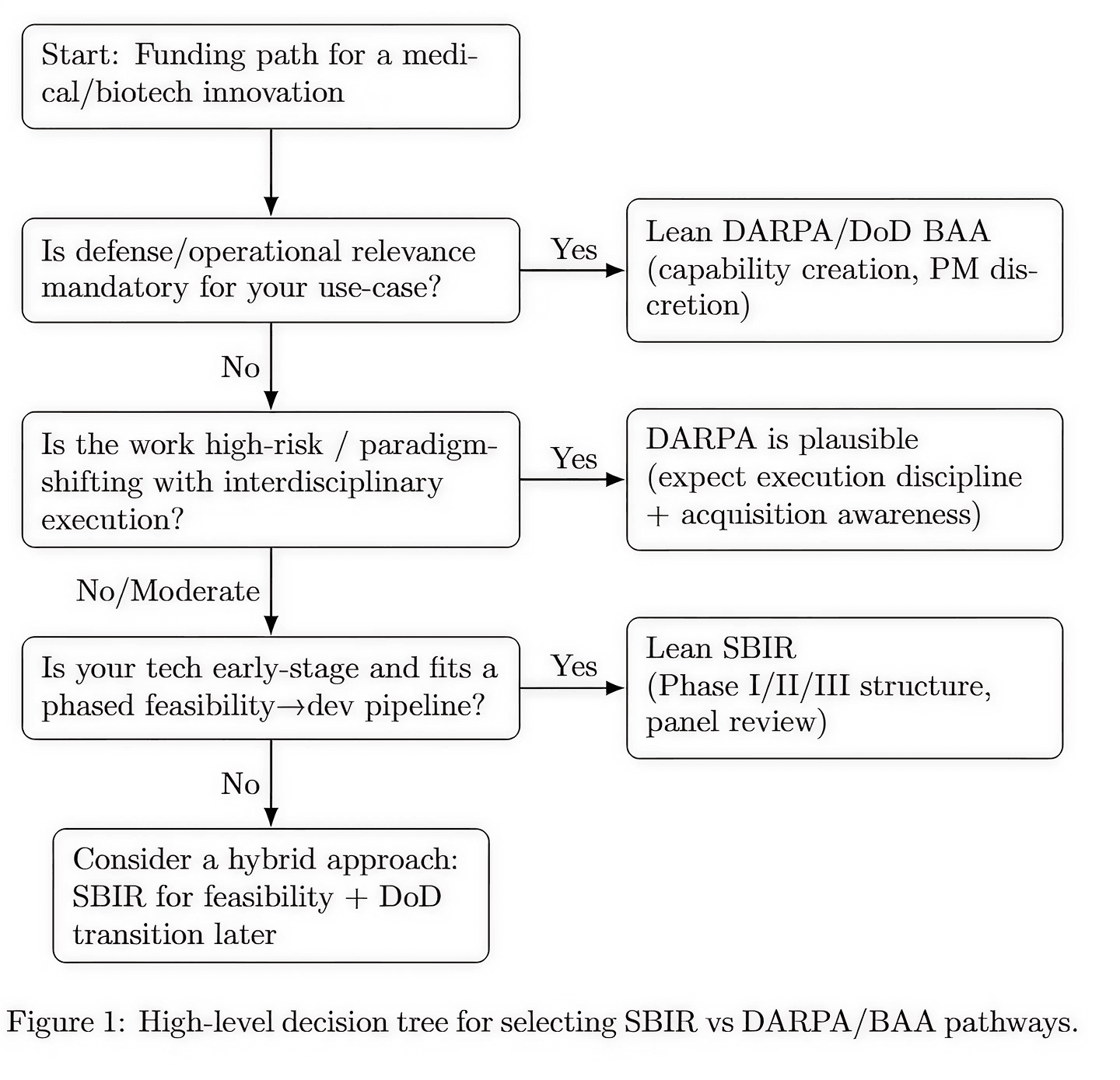
Common Failure Modes When Choosing the Wrong Path
Many medical companies fail not because their technology is weak, but because they pursue the wrong funding mechanism.
SBIR proposals frequently fail when companies oversell transformational impact without sufficient preliminary data. DARPA proposals fail when companies present excellent science but cannot articulate operational relevance, execution discipline, or acquisition awareness.
Treating DARPA like NIH or SBIR is one of the fastest ways to lose credibility with evaluators. Conversely, forcing a DARPA-style pitch into an SBIR framework often results in rejection for being “overly ambitious” or “insufficiently focused.”
How ProposalWin Advises Medical and Biotech Clients
At ProposalWin, we help medical and biotech firms make this decision before they invest time and capital into the wrong proposal. Our approach integrates scientific positioning with acquisition strategy, ensuring that the funding path supports, not constrains, long-term growth.
We routinely assist clients in determining whether SBIR, DARPA, or a hybrid pathway best aligns with their technology and commercialization goals. For DARPA-aligned companies, we develop BAA response strategies that integrate medical credibility with defense relevance. For SBIR-focused firms, we optimize phase progression and evaluator alignment.
Because our team understands both clinical science and federal contracting, we act as translators between innovation and execution.
Final Thoughts
SBIR and DARPA are not competing programs. They are tools designed for fundamentally different types of medical innovation. Choosing the right one can determine whether a promising technology stalls at the proposal stage or scales into a nationally significant capability.
Medical and biotech companies that understand this distinction early gain a decisive advantage, not only in funding, but in strategic positioning across the federal ecosystem.
If your organization is evaluating SBIR or DARPA funding and wants a clear, defensible strategy, ProposalWin is equipped to guide that decision.

Ready to Submit Stronger, Fully Compliant Proposals?
Join the agencies and contractors who trust ProposalWin to deliver faster, better, compliant proposals, and see how much smoother your next bid can be.


